PGDIS Position Statement on the Transfer of Mosaic Embryos 2021
- Report of PGDIS Expert Consultation on Mosaic Embryo Transfer, August 19, 2021(Click for PDF Document)
- SUMMARY
- Chromosome testing strategies, such as PGT-A, improve initial IVF outcomes by avoiding unwitting transfer of aneuploid embryos in morphology-based selection practices. Newer technologies have revealed that some embryos may appear to have intermediate whole chromosome (or parts of a chromosome termed segmental) copy number results suggesting trophectoderm (TE) mosaicism. An embryo with a TE mosaic-range result (to be referred to as “mosaic embryos” in the rest of this document) may be the only option for transfer for some patients. Recent data suggests that such mosaic embryos can be transferred without added risk of abnormal birth outcomes. Some published research suggests that mosaic embryo transfers are associated with increased implantation failure and miscarriage rates (Figure 1) with higher values of mosaicism appearing to be less favorable for producing good outcomes for the patient although there are only a few controlled studies examining this possibility. Transfer of lower range mosaic embryos have variable reports with some groups suggesting outcomes similar to euploid embryos (Capalbo et al., 2021), whereas other groups report higher pregnancy failure rates ( Wang et al., 2021). Data are still limited regarding outcome of mosaic embryo transfers, but information on outcomes with follow up exists on over 1000 cases.
In this Position Statement we provide guidance to laboratories, clinics, clinicians and counsellors to assist in discussions regarding the utility and transfer of mosaic embryos.
- INTRODUCTION
- As a society, PGDIS promotes the implementation of quality processes at all stages of embryo analysis including technical competency in performing any PGT process as well as appropriate interpretation of all testing results. Since the release of the previous Preimplantation Genetic Diagnosis International Society (PGDIS) Position Statement 2019 (www.pgdis.org), there has remained uncertainty about implications of mosaic embryo transfer. The purpose of this document is to review the most recent information available and provide an updated summary to test laboratories, clinics, clinicians and genetic counsellors regarding the transfer of mosaic embryos, replacing previous 2016 (https://pgdis.org/docs/newsletter_052417.html) and 2019 (Cram et al., 2019) documents issued on behalf of PGDIS.
- BACKGROUND
- The primary purpose of preimplantation genetic testing for aneuploidy (PGT-A) is to improve selected IVF transfer outcomes by reducing the number and impact of aneuploid embryos inherent in morphology-based embryo transfer choices (Forman et al., 2013). Transfer of a euploid embryo has demonstrated improved rates for implantation, pregnancy and live birth over aneuploid embryos (Tiegs et al., 2021; Wang et al., 2021).
A majority of early studies failed to demonstrate an advantage of PGT-A performed using cleavage stage biopsy with limited FISH analysis. Comprehensive chromosome analysis (Fragouli et al., 2008) in conjunction with biopsy of several trophectoderm cells is now considered optimal for evaluation of embryo chromosomal status. Earlier stage biopsy and its greater potential for reduction in embryo outcomes has been discontinued in most clinics. Analysis of more than one cell in a single assay however, introduces the possibility of whole chromosome (or partial/segmental chromosome) intermediate copy number results.
- OVERVIEW OF NEW KNOWLEDGE
- Incidence of mosaic embryos
- Chromosome mosaicism has been observed commonly although usually in only a minority of embryos. Sensitive technologies such as array CGH and NGS based methods can variably distinguish uniform aneuploidies (affecting all cells in the biopsy) from mosaic aneuploidies (affecting only some of the cells in the biopsy). At the blastocyst stage, the incidence of reported mosaicism using NGS methods is highly variable amongst clinics, ranging from as low as 2% to as high as 40% with the vast majority of clinics reporting between 5-15% depending on age group being investigated (Fragouli et al., 2019; Munne et al., 2016; Rodrigo et al., 2020; Ruttanajit et al., 2016). A consistent high incidence of mosaic embryos in a selected clinic may be related to predominant patient age group, clinical treatment and/or specific embryology practices (Fragouli et al., 2019) while a high level of apparent mosaicism across all referral clinics may be indicative of poorer testing laboratory practices. In either case, a review of clinical and/or testing laboratory practices may be warranted. Clinics sending biopsies for PGT-A to an outside testing laboratory should request the laboratory to disclose their embryo mosaic rates and cut off ranges. This will assist clinics in assessing their own performances as well as the analytical capabilities of any referred testing laboratory.
- Transfer outcomes from mosaic embryos
- Since the first published study reporting successful pregnancies after transfer of mosaic embryos (Greco et al., 2015), other groups have also reported outcomes involving larger numbers of mosaic embryos (Munne et al., 2017; Victor et al., 2019a; Viotti et al., 2021; Zhang et al., 2019; Zore et al., 2019). These studies have revealed that mosaic or mosaic segmental embryos do give rise to healthy pregnancies (Tiegs et al., 2021; Viotti et al., 2021) but suggest such transfers may be associated with reduced implantation rates and often higher miscarriage rates (Wang et al., 2021).
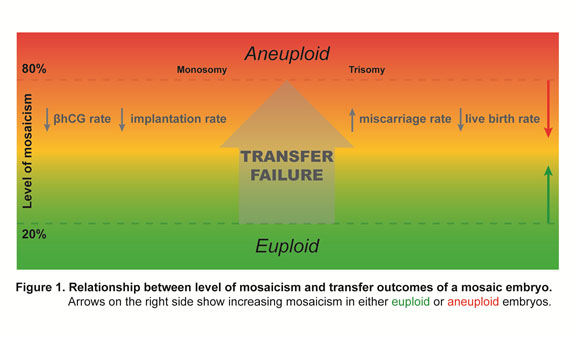
The collective transfer data still only comprises approximately 1000 mosaic embryos but it is evident that a high proportion of mosaic embryos have a significant level of developmental competence and should not be disregarded in terms of suitability for transfer. In general, good success rates were achieved after the transfer of lower range mosaic embryos, whereas apparently mosaic embryos that appeared to have higher levels of abnormal cells in the TE biopsy specimen were less likely to achieve a viable pregnancy. At present, nearly all prenatal diagnoses of established pregnancies after a mosaic embryo transfer have revealed normal euploid fetuses with all live births reported to date showing no evidence of chromosome-based syndromes. Currently, there have been only a few isolated reports on mosaic embryo transfers giving rise to a non-syndromic very low level mosaic child (Kahraman, et al., 2020) or an affected child (Mounts E., 2019).
More recently, the total analysis of mosaic embryos donated to research has revealed additional information on the chromosomal constitution of mosaic blastocysts (Viotti et al., 2021). In general, a high level of mosaicism in the initial biopsy often shows a full aneuploidy in subsequent trophectoderm biopsy and ICM. However, if a lower level of mosaicism was followed in subsequent trophectoderm and ICM biopsies, many embryos were uniformly euploid (Marin et al., 2021; Ou et al., 2020; Victor et al., 2019b).
- How does this affect aneuploidy testing in clinical practice?
- Although most (>85%) TE biopsy results are either uniform euploid for all chromosomes or full aneuploid involving one or more chromosomes, a small proportion of embryo biopsies may show intermediate copy number changes for one or more chromosomes. Although mosaicism detected in TE biopsies can theoretically have clinical implications for the fetus and/or placenta in any pregnancy, including effects on placental function and/or liveborn disease syndromes (Grati et al., 2018), transfer of mosaic embryos may be considered after appropriate counselling of the patient and discussion of alternatives. The actual risk compared to natural pregnancies is small (Viotti et al., 2021) with differences observed likely representing true mosaic transfers amongst minor TE mosaics or artefactual analyses.
- FOR THE TESTING LABORATORY
- Circumstantial evidence is emerging that suggests that NGS and associated data analysis pipelines used to measure chromosome copy number may at times incorrectly indicate mosaicism (Fragouli et al., 2019; Marin et al., 2021).
Theoretically, mosaicism estimates could be exaggerated by the following:
(i) Poor biopsy technique
(ii) Poor sample handling/transport
(iii) Sub-optimal DNA amplification and library construction
(iv) Choice of algorithms used for normalizing the chromosome mapping bins
FURTHER COMMENTS
1. For technical reasons, only an analysis platform that can reproducibly measure copy number should be used for reporting mosaic levels in the biopsy sample. Testing laboratories can perform their own baseline control experiments for both euploid and aneuploid amplified DNA products from a range of samples. Such experiments may be repeated at regular intervals, to be defined within each laboratory and to ensure mosaicism detection does not alter. Published works from different groups suggest a typical cut off value for euploid assignment is <20% and for aneuploidy assignment >80%. These values essentially represent noise bands and may show some variation based on the specific technology or algorithms used (Fragouli et al., 2017; Maxwell et al., 2016; Munne et al., 2017; Spinella et al., 2018). Embryos in the lower ‘mosaic’ range value may be reported as euploid, whereas embryos in the upper ‘mosaic’ value may be reported as aneuploid. Profiles with chromosome values outside these ranges are considered to indicate potential mosaicism. Some groups use less stringent euploid/aneuploid cut off values, resulting in lower reported intermediate copy numbers but accepting higher analytical noise levels with its implications for overlap in the mosaic range. Groups that report higher mosaic embryo transfer outcomes may be reflecting higher false rates of mosaic calls associated with different testing platforms and/or algorithms used in analysis (Navratil, et al., 2020; Rodrigo et al., 2020; Zhou, et al., 2021). In either case, these upper and lower cutoff ranges should be reported by the testing laboratory to the referring clinician in order to facilitate any transfer discussions with their patient.
2. Given the nature of the biology underlying the genesis and propagation of mosaicism, a TE biopsy suggested to be mosaic may not accurately reflect the rest of the embryo. Logically, a fixed range stating high/low mosaicism for the entire embryo should not be provided.
3. There is inherent difficulty in assigning a single average value to what may actually be a relatively broad variation along a single chromosome in addition to an analytical error as with euploid/aneuploid ranges. This means any value should therefore be considered a reference point for reporting purposes only.
4. While it is understood that commercial imperatives may be involved, testing laboratories should not classify mosaic embryos as fully aneuploid since this may reduce patient cycle potential. This includes embryos with multiple chromosomes in the mosaic range. This may mean a “No Result” assignment is most appropriate (Marin et al., 2021).
5. Testing laboratories should refrain from classifying a mosaic embryo as suitable or not suitable for transfer since this may restrict clinical treatment options
6. Laboratory report formats should be updated to include reporting of
mosaic results, apparent % mosaicism, any cut off values used and any chromosome abnormality identified.
7. A chromosome result profile that indicates apparent mosaicism for any patient embryo should also be provided on request for the purpose of genetic counsellors or clinicians explaining the PGT-A results to patients
- FOR THE IVF CLINIC
- It is recommended that 5- 10 TE cells be biopsied in order to minimize the impact of the process on the remaining embryo while still giving a robust, balanced amplification- care should be taken at all times to ensure minimum effect on the embryo. Damage to the cells during biopsy, as well as washing and loading should be minimized to reduce amplification bias and yield a DNA product reflecting the original embryonic cells. If there is a consistently high incidence of mosaicism identified in embryo cohorts within a given clinic, consideration should be given to investigating both the embryology and PGT-A practice so as to identify any possible underlying explanations.
- FOR THE GENETIC COUNSELLOR/CLINICAL SUPPORT
- The wide variety and quality of information both in the scientific literature and social media forums has confused the understandings of the usefulness of mosaic embryos in IVF treatment. Debate in the scientific literature, popular press and on social media has led to confusion, not only amongst the clinics but also amongst patient support and advocacy groups. Preimplantation Genetic Testing for Aneuploidy (PGT-A) is a process designed to improve the outcome of any specific transfer by identifying those embryos which have a chromosome constitution most likely to lead to a successful transfer outcome.
Pregnancy failure with aneuploid embryos is undisputed, as is necessity for a balanced chromosome set for a successful pregnancy is also undisputed. At this point in time, the best approach for examining the constitutional chromosome set is to remove a small sample from the blastocyst stage embryo. As with any analysis involving more than one cell, the possibility of identifying chromosomal differences within those cells exists. If there are two different chromosome complements amongst the cells, the chromosome(s) will show an intermediate copy number profile, i.e. neither two (termed euploid), nor one or three (termed aneuploid). Presence of an intermediate level is referred to as a mosaic (mixed) state. Since only a small piece is removed from the embryo, a mosaic state may be limited to the region biopsied or may be present throughout other parts of the embryo.
Any individual embryo cohort may have no transferrable embryo, or may have several embryos classified as mosaic. This Position Statement is devised to assist in the decision-making process when faced with the option of a mosaic embryo transfer. The most recent report summarizing outcomes after 1,000 mosaic embryo transfers (Viotti et al., 2021) suggests that while implantation potential may be reduced, the risks of any subsequent births being chromosomally abnormal are low.
It is understood that different opportunities and constraints are faced by each individual patient; thus, the decision-making process needs to be fully discussed with an appropriate professional. It should be kept in mind that mosaic embryos have always existed and been used in the IVF process without being identified as such. It should also be recognized that some of the euploid embryos selected for transfer could prove mosaic if biopsied in another region of the trophectoderm.
Given the nature of mosaicism and the way in which it arises during early embryonic development, it is obvious that a single biopsy specimen characterized as mosaic does not prove that the surrounding trophectoderm or the rest of the embryo is also mosaic. Increasing level of mosaicism may be less favourable to good outcomes (FIGURE 1) but for both technical reasons (analysis platform, amplification variations, analysis algorithms) and biological reasons (localized mosaicism vs uniform mosaicism) no precise cut-off values for transfer should be assigned (Lin, et al., 2020; Marin et al., 2021).
- RECOMMENDATIONS FOR THE CLINICIAN
- While laboratories deliver reports for individual embryos, clinicians should have some proficiency in understanding an embryo chromosome result profile. Clinicians may be called upon by patients to explain transfer opportunities. A chromosomal profile can usually be presented as a simple, pictorial representation of an embryo’s relative chromosome copy number.
1. Patients should continue to be advised that any genetic test based on sampling one or small number of cells biopsied from preimplantation embryos cannot be 100% accurate because of a combination of technical and biological factors, including chromosome mosaicism.
2. Patient information and consent forms for aneuploidy testing should be modified to include the possibility of mosaic results.
3. In general, transfer of blastocysts with a normal euploid result should be prioritized over those with mosaic results unless other indications, such as patient preference are raised.
4. Transferring a mosaic embryo is not without increased risk compared to the transfer of a euploid embryo. In considering the transfer of a mosaic blastocyst, the following options can be discussed with the patient:
(i) A further PGT-A cycle to increase the chance of identifying a euploid blastocyst for transfer.
(ii) Or transfer the mosaic blastocyst after appropriate consultation, regarding the potential risks.
PRENATAL OPTIONS
Prenatal diagnosis of an established pregnancy after euploid or mosaic PGT is recommended by PGDIS. This is consistent with current ACOG/ACMG recommendations that state prenatal diagnosis should be discussed and made available for every pregnancy, regardless of method of conception or prior genetic testing.
Non Invasive Testing
For early pregnancy investigations, preference should be given to 24 chromosome NIPT methodology that includes the mosaic chromosome(s) in question- the simple 5 chromosome NIPT tests (21, 18, 13, X and Y) available in many countries may not be appropriate for some specific investigations. It should be noted that segmental aneuploidy detected by PGT-A may be below limits of detection by NIPT. The ordering provider must be aware as to whether the segment is within detection limits. The requester should also understand that non-invasive testing can only assess placental chromosome status which does not always reflect the remaining structures or the fetus.
Invasive Testing
Amniocentesis analysis from gestation week 14 onwards is considered to be the most representative of the chromosomal complement of the fetus. Earlier gestational stage, chorion villus sampling (CVS), may be considered but, as with NIPT, it may only reveal placental chromosome constitution which could differ from the fetal chromosome set. Non-directive counselling regarding all options should be offered in all cases.
- SUGGESTED RECOMMENDATIONS TO ASSIST IN THE PRIORITIZATION OF MOSAIC EMBRYOS CONSIDERED FOR TRANSFER
- Based on new knowledge gained from recent embryo analysis and transfer studies, the following is a guide to assist the clinician (or a genetic counsellor if available) when a mosaic embryo is being considered for transfer:
1. Embryos with higher-level mosaicism may be associated with less favorable outcomes compared to lower-level mosaicism. Currently there is little experience on high grade mosaic embryo transfers. Relative percentage of mosaicism seems to be a better predictor of outcome than the specific chromosome(s) involved and thus should be included in reporting and patient discussion.
2. A decision to transfer a mosaic embryo can be prioritized either on the level of mosaicism or type of mosaicism (whole chromosome vs segmental changes). If there is a choice between the transfer of two embryos with similar levels of mosaicism, preference may be considered based on embryo morphology, giving higher morphological grades to be tend to give better outcomes or on the nature of the variation (segmental mosaic embryo transfers are reported to give outcomes more similar to euploid embryos than are whole chromosome mosaic embryo transfers).
- IN CONCLUSION
- Developments in genomic technologies for PGT have allowed more complete spectrum of chromosome abnormalities to be identified, including full chromosome and segmental mosaicism- areas in which current knowledge of the outcomes is incomplete and still evolving. Historical IVF outcomes, where transfer of mosaic embryos was inevitable, have not indicated increased risks for live born chromosome disorders compared to natural pregnancies. Transfer of mosaic embryos seems to be a relatively safe option for couples, with low or minimal risk of negative outcomes for the birth beyond the background risk for any pregnancy.
NIPT has been shown capable of detecting many even rare (non-live born) trisomies (Scott et al., 2018). Non-invasive follow up of the original trophectoderm mosaicism result is thus now available. A traditional invasive test is also available but at a later gestational time.
At the research level, chromosome analysis of donated mosaic embryos continues to shed light on the significance of the initial biopsy assessments and gives valuable information about the genetic constitution of mosaic embryos. Similarly, detailed chromosome investigations of the placenta after birth would add valuable information on the nature and extent of any mosaicism observed in the original transferred mosaic embryo. As further information evolved, this Position Statement will be updated accordingly.
- REFERENCES
- Capalbo, A., Poli, M., Rienzi, L., Girardi, L., Cimadomo, D., Benini, F., Farcomeni, A., Cuzzi, J., Rubio, C., Albani, E., Sacchi, L., Vaiarelli, A., Vogel, I., Hoffmann, E., Livi, C., Levi-Setti, P.E., Ubaldi, F.M. and Simón, C. A prospective double-blinded non-selection trial of reproductive outcomes and chromosomal normalcy of newborns derived from putative low/moderate-degree mosaic IVF embryos. medRxiv 2021:2021.2002.2007.21251201. doi:10.1101/2021.02.07.21251201
Cram, D.S., Leigh, D., Handyside, A., Rechitsky, L., Xu, K., Harton, G., Grifo, J., Rubio, C., Fragouli, E., Kahraman, S., Forman, E., Katz-Jaffe, M., Tempest, H., Thornhill, A., Strom, C., Escudero, T., Qiao, J., Munne, S., Simpson, J.L. and Kuliev, A. PGDIS Position Statement on the Transfer of Mosaic Embryos 2019. Reprod. Biomed. Online. 2019; 39 Suppl 1:e1-e4. doi:10.1016/j.rbmo.2019.06.012
Forman, E.J., Upham, K.M., Cheng, M., Zhao, T., Hong, K.H., Treff, N.R. and Scott, R.T., Jr. Comprehensive chromosome screening alters traditional morphology-based embryo selection: a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer. Fertil. Steri.l 2013; 100:718-724. doi:10.1016/j.fertnstert.2013.04.043
Fragouli, E., Alfarawati, S., Spath, K., Babariya, D., Tarozzi, N., Borini, A. and Wells, D. Analysis of implantation and ongoing pregnancy rates following the transfer of mosaic diploid-aneuploid blastocysts. Hum. Genet. 2017; 136:805-819. doi:10.1007/s00439-017-1797-4
Fragouli, E., Lenzi, M., Ross, R., Katz-Jaffe, M., Schoolcraft, W.B. and Wells, D. Comprehensive molecular cytogenetic analysis of the human blastocyst stage. Hum. Reprod. 2008; 23:2596-2608. doi: 10.1093/humrep/den287.
Fragouli, E., Munne, S. and Wells, D. The cytogenetic constitution of human blastocysts: insights from comprehensive chromosome screening strategies. Hum. Reprod. Update. 2019; 25:15-33. doi:10.1093/humupd/dmy036
Grati, F.R., Gallazzi, G., Branca, L., Maggi, F., Simoni, G. and Yaron, Y. An evidence-based scoring system for prioritizing mosaic aneuploid embryos following preimplantation genetic screening. Reprod. Biomed. Online. 2018; 36:442-449. doi:10.1016/j.rbmo.2018.01.005
Greco, E., Minasi, M.G. and Fiorentino, F. Healthy Babies after Intrauterine Transfer of Mosaic Aneuploid Blastocysts. N. Engl. J. Med. 2015; 373:2089-2090. doi:10.1056/NEJMc1500421
Kahraman, S., Cetinkaya, M., Yuksel, B., Yesil, M. and Pirkevi Cetinkaya, C. The birth of a baby with mosaicism resulting from a known mosaic embryo transfer: a case report. Hum. Reprod. 2020; 35:727-733. doi:10.1093/humrep/dez309
Lin, P.Y., Lee, C.I., Cheng, E.H., Huang, C.C., Lee, T.H., Shih, H.H., Pai, Y.P., Chen, Y.C. and Lee, M.S. Clinical Outcomes of Single Mosaic Embryo Transfer: High-Level or Low-Level Mosaic Embryo, Does it Matter? J Clin Med 2020; 9. doi:10.3390/jcm9061695
Marin, D., Xu, J. and Treff, N.R. Preimplantation genetic testing for aneuploidy: A review of published blastocyst reanalysis concordance data. Prenat. Diagn. 2021; 41:545-553. doi:10.1002/pd.5828
Maxwell, S.M., Colls, P., Hodes-Wertz, B., McCulloh, D.H., McCaffrey, C., Wells, D., Munne, S. and Grifo, J.A. Why do euploid embryos miscarry? A case-control study comparing the rate of aneuploidy within presumed euploid embryos that resulted in miscarriage or live birth using next-generation sequencing. Fertil. Steril. 2016; 106:1414-1419 e1415. doi:10.1016/j.fertnstert.2016.08.017
Mounts, E.L., Zhu, S., Sanderson, R., Coates, A. and Hesla, JS. Mosaic embryo diagnosis correlated with abnormal 15q Duplication Syndrome in offspring. Fertil. Steril. 2019; 112 Suppl :e241-242. doi:10.1016/j.fertnstert.2019.07.1375
Munne, S., Blazek, J., Large, M., Martinez-Ortiz, P.A., Nisson, H., Liu, E., Tarozzi, N., Borini, A., Becker, A., Zhang, J., Maxwell, S., Grifo, J., Babariya, D., Wells, D. and Fragouli, E. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil. Steril. 2017; 108:62-71 e68. doi:10.1016/j.fertnstert.2017.05.002
Munne, S., Grifo, J. and Wells, D. Mosaicism: "survival of the fittest" versus "no embryo left behind". Fertil Steril 2016;105:1146-1149. doi:10.1016/j.fertnstert.2016.01.016
Navratil, R., Horak, J., Hornak, M., Kubicek, D., Balcova, M., Tauwinklova, G., Travnik, P. and Vesela, K. Concordance of various chromosomal errors among different parts of the embryo and the value of re-biopsy in embryos with segmental aneuploidies. Mol. Hum. Reprod. 2020; 26:269-276. doi:10.1093/molehr/gaaa012
Ou, Z., Chen, Z., Yin, M., Deng, Y., Liang, Y., Wang, W., Yao, Y. and Sun L. Re-analysis of whole blastocysts after trophectoderm biopsy indicated chromosome aneuploidy. Hum Genomics. 2020; 14:3. Doi:1186/s40246-019-0253-z.
Rodrigo, L., Clemente-Ciscar, M., Campos-Galindo, I., Peinado, V., Simon, C. and Rubio, C. Characteristics of the IVF Cycle that Contribute to the Incidence of Mosaicism. Genes (Basel). 2020; 11. doi:10.3390/genes11101151
Ruttanajit, T., Chanchamroen, S., Cram, D.S., Sawakwongpra, K., Suksalak, W., Leng, X., Fan, J., Wang, L., Yao, Y. and Quangkananurug, W. Detection and quantitation of chromosomal mosaicism in human blastocysts using copy number variation sequencing. Prenat. Diagn. 2016; 36:154-162. doi:10.1002/pd.4759
Scott, F., Bonifacio, M., Sandow, R., Ellis, K., Smet, M.E. and McLennan, A. Rare autosomal trisomies: Important and not so rare. Prenat. Diagn. 2018; 38:765-771. doi:10.1002/pd.5325
Spinella, F., Fiorentino, F., Biricik, A., Bono, S., Ruberti, A., Cotroneo, E., Baldi, M., Cursio, E., Minasi, M.G. and Greco, E. Extent of chromosomal mosaicism influences the clinical outcome of in vitro fertilization treatments. Fertil. Steril. 2018; 109:77-83. doi:10.1016/j.fertnstert.2017.09.025
Tiegs, A.W., Tao, X., Zhan, Y., Whitehead, C., Kim, J., Hanson, B., Osman, E., Kim, T.J., Patounakis, G., Gutmann, J., Castelbaum, A., Seli, E., Jalas, C. and Scott, R.T., Jr. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing-based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil. Steril. 2021; 115:627-637. doi:10.1016/j.fertnstert.2020.07.052
Victor, A.R., Tyndall, J.C., Brake, A.J., Lepkowsky, L.T., Murphy, A.E., Griffin, D.K., McCoy, R.C., Barnes, F.L., Zouves, C.G. and Viotti, M. One hundred mosaic embryos transferred prospectively in a single clinic: exploring when and why they result in healthy pregnancies. Fertil. Steril. 2019a; 111:280-293. doi:10.1016/j.fertnstert.2018.10.019
Victor, A.R., Griffin, D.K., Brake, A.J., Tyndall, J.C., Murphy, A.E., Lepkowsky, L.T., Lal, A., Zouves, C.G., Barnes, F.L., McCoy, R.C. and Viotti, M. Assessment of aneuploidy concordance between clinical trophectoderm biopsy and blastocyst. Hum. Reprod. 2019b;34:181-192. doi:10.1093/humrep/dey327
Viotti, M., Victor, A.R., Barnes, F.L., Zouves, C.G., Besser, A.G., Grifo, J.A., Cheng, E.H., Lee, M.S., Horcajadas, J.A., Corti, L., Fiorentino, F., Spinella, F., Minasi, M.G., Greco, E. and Munne, S. Using outcome data from one thousand mosaic embryo transfers to formulate an embryo ranking system for clinical use. Fertil. Steril. 2021; 115:1212-1224. doi:10.1016/j.fertnstert.2020.11.041
Wang, L., Wang, X., Liu, Y., Ou, X., Li, M., Chen, L., Shao, X., Quan, S., Duan, J., He, W., Shen, H., Sun, L., Yu, Y., Cram, D.S., Leigh, D. and Yao, Y. IVF embryo choices and pregnancy outcomes. Prenat. Diagn. 2021. doi:10.1002/pd.6042
Zhang, L., Wei, D., Zhu, Y., Gao, Y., Yan, J. and Chen, Z.J. Rates of live birth after mosaic embryo transfer compared with euploid embryo transfer. J. Assist. Reprod. Genet. 2019; 36:165-172. doi:10.1007/s10815-018-1322-2
Zhou, S., Xie, P., Zhang, S., Hu, L., Luo, K., Gong, F., Lu, G. and Lin, G. Complex mosaic blastocysts after preimplantation genetic testing: prevalence and outcomes after re-biopsy and re-vitrification. Reprod. Biomed. Online. 2021; 43:215-222. doi:10.1016/j.rbmo.2021.04.006
Zore, T., Kroener, L.L., Wang, C., Liu, L., Buyalos, R., Hubert, G. and Shamonki, M. Transfer of embryos with segmental mosaicism is associated with a significant reduction in live-birth rate. Fertil Steril 2019;111:69-76. doi:10.1016/j.fertnstert.2018.08.057
- KEYWORDS
- good laboratory practice, guidelines, PGD, PGDIS, protocol, quality assurance
PGDIS 2019 Position Statement
- The Preimplantation Genetic Diagnosis International Society (PGDIS) guidelines for good practice in PGD: program requirements and laboratory quality assurance (RBMOnline 2008; Vol 16, No 1)
- ABSTRACT
- The Preimplantation Genetic Diagnosis International Society (PGDIS) was organized in October 2002, with the purpose of encouraging and coordinating research, education and training in this multidisciplinary field, requiring a close collaboration of obstetricians, fertility specialists, embryologists and human geneticists. One of the major tasks of PGDIS is to advance the safety and accuracy of PGD and to encourage its adoption into clinical practice for improvement of genetic practices and reproductive medicine. In this context, PGDIS published voluntary guidelines applicable for any center offering PGD in 2004, and these guidelines are now being updated and extended based on the present extensive PGD experience. The application of these guidelines is intended to further benefit patients and provide guidance to the laboratory staff. As in previous guidelines, PGDIS presents this document being aware that differences in national regulations exist that can affect local PGD practice. The document contains recent consensus points of general application that promote quality biopsy procedures and laboratory practice, enabling PGD centers to offer an improved clinical outcome to their patients. A variety of aspects related to a safe working system have been taken into consideration, based on the assumption that a quality program depends on the cooperation of the whole PGD team.
- KEYWORDS
- good laboratory practice, guidelines, PGD, PGDIS, protocol, quality assurance

